Tinctures plants in alcoholic solutions or inhaled via steam from boiling suspensions of the parts. A NH3 B PH3 C AsH3 D SbH3 3.

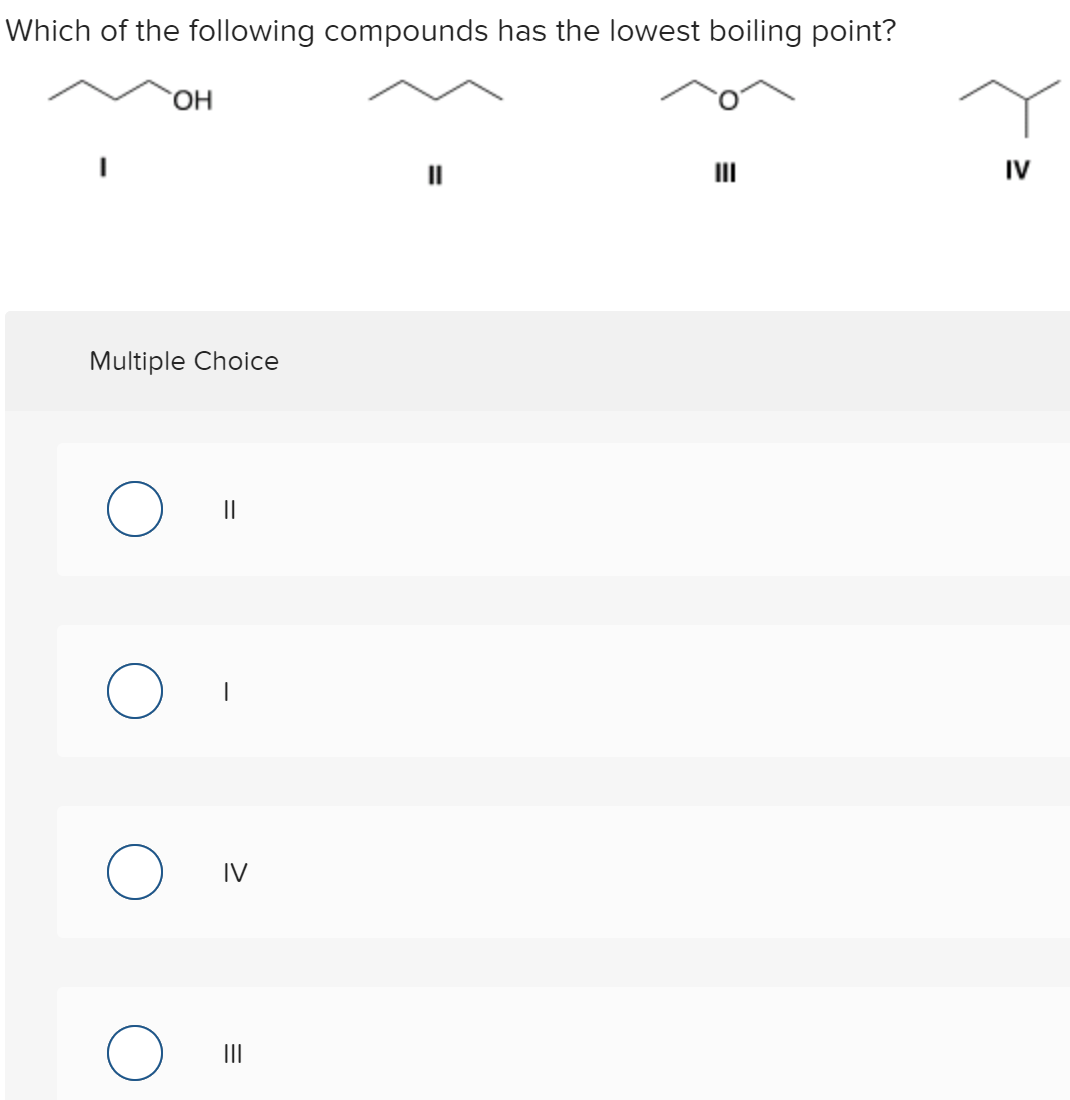
Solved 1 Which Of The Following Compounds Has The Lowest Chegg Com
Gas chromatography in combination with olfactometric techniques GC-O can help to detect potent odorants without knowing their.
. Sodium nitrate - PRES REG 500 ppm - Alone or w sodium nitrite as a preservative and color fixative in smoked cured salmon shad. The application of GC-MS in this area has marked a real turning point for flavor research. The boiling point is an important property because it determines the speed of evaporation.
Mildly acidic it requires careful handling because it can cause chemical burns. Scientific analysis of plant components follows a logical pathway. Which of the following should have the lowest boiling point.
If a nonvolatile solute lowers the vapor pressure of a solvent it must also affect the boiling point. In the following discussion we must therefore keep the chemical nature of the solute firmly in mind. Recall that the normal boiling point of a substance is the temperature at which the vapor pressure equals 1 atm.
Phenol was first extracted from coal tar but today is produced on a. By thinking about noncovalent intermolecular interactions we can also predict relative melting points. Poultices can also be made from concentrated teas or tinctures 30 224.
Stronger intermolecular interactions result in a higher melting point. Since organic compounds tend to have more hydrogen atoms than inorganic compounds this makes organic compounds typically. Ionic compounds as expected.
It is a white crystalline solid that is volatileThe molecule consists of a phenyl group C 6 H 5 bonded to a hydroxy group OH. Based on their structures rank phenol benzene benzaldehyde and benzoic acid in terms of lowest to highest boiling point. Dried plant parts can be added to oils or petroleum jelly and applied externally.
A C5H12 B C. Hydrogen has a very low density. Small amounts of low-boiling-point solvents like diethyl ether dichloromethane or acetone will evaporate in seconds at room temperature while high-boiling-point solvents like water or dimethyl sulfoxide need higher temperatures an air flow or the application of vacuum for fast evaporation.
Because the vapor pressure of. Select the pair of compounds in which the substance with the higher vapor pressure at a given temperature is listed first. This group of compounds has received a great deal of.
BC REG GMP Boiler water additive -173310. PS by USDA - As a source of nitrite w or wo sodium or potassium nitrite. In fact its the lowest density atom.
Which of the following has a boiling point which does not fit the general trend. Phenol also called carbolic acid is an aromatic organic compound with the molecular formula C 6 H 5 OH. All of the same principles apply.
A C7H16 C5H12 B CCl4 CBr4 C H2O. Indeed the number of known flavors has increased to over 7000 compounds however there is no information about odor active components.

Which Of The Following Compound Has Lowest Boiling Point Youtube
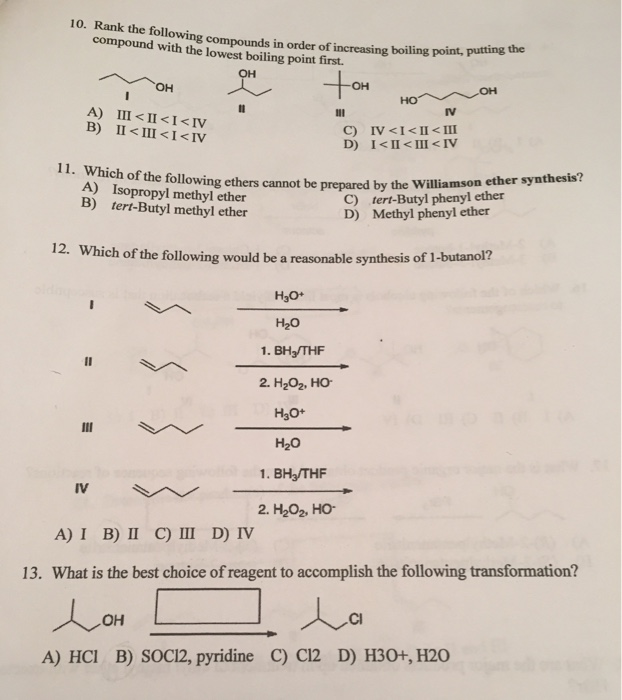
Solved 9 Which Of The Following Compounds Has The Highest Chegg Com
0 Comments